
Photo by anttler via flickr
Designing organic molecules that perform repeated mechanical motions is not easy. The molecule needs to be robust on the one hand, and on the other hand have different stable states between which it can alternate. Achieving such complex functionality requires careful design considerations. Nature has solved this problem, and molecular motors perform important functions in living organisms, for example in enzymes.
The design and synthesis of artificial mechanical molecules performing specific functions remains a challenge. For example, molecular rotors have been fabricated before, but only showed limited functionality: their direction of rotation could not be reversed.
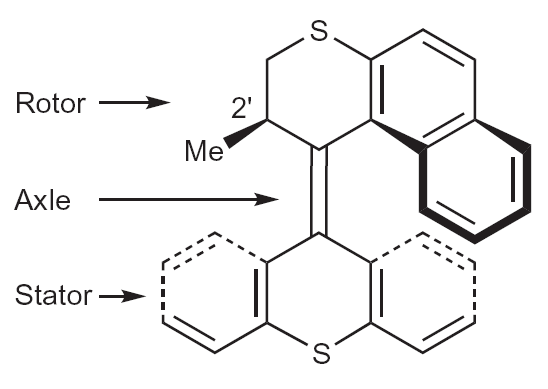
The concept of a molecular rotor. The rotor is connected to the stator via an alkene bond that acts as axle. Note that the rotor part used by Feringa et al. differs slightly from this example. Reprinted by permission from Macmillan Publishers Ltd. Nature Chemistry, doi: 10.1038/nchem.872 (2010).
Ben Feringa and his colleagues from the University of Groningen in the Netherlands have now realized the first synthetic molecular rotor that can reverse its direction. They synthesized an organic molecule consisting of two parts, a rotor and a stator section, which are connected through a chemical bond that acts as an axle. The rotation of the rotor occurs in four steps and is triggered by light.
To reverse the direction of the rotation, an ionized hydrogen atom (a proton) is attached to the rotor. This changes the stereochemistry of the molecule and hence the default orientation of the rotation without affecting the actual rotation itself. Removing the proton reverts the molecule back to its original rotation.
The subtle control that Feringa and colleagues achieve points to a sophisticated control over mechanical function of organic molecules. In particularly the change in functionality as a response to an external stimulus — in this case it is a change in pH value — could lead to interesting molecular functionality, write Feringa and colleagues in their Nature Chemistry paper: “The stage is now set to couple the change in directionality to the modulation of a specific function.” More complex molecular machines await.
Reference:
Ruangsupapichat, N., Pollard, M., Harutyunyan, S., & Feringa, B. (2010). Reversing the direction in a light-driven rotary molecular motor Nature Chemistry DOI: 10.1038/nchem.872


November 1, 2010
Chemistry, Nanotechnology