Your desk at work, is it chaotic as mine, or clean and ordered? If the latter, I salute you, because it takes work to keep a desk tidy. Otherwise, chaos will soon reign. And while I admit that I should keep my desk cleaner (and no, I won’t share photos here), I have an excellent excuse: it is a fundamental law of nature that disorder and chaos are always increasing.
A measure of this disorder is a quantity called entropy. To clean your desk it takes work, which creates heat, which is energy that is wasted on the environment. So even though the entropy of the desk is reduced, overall it increases. And this is the second law of thermodynamics: on average, the entropy in the universe is always increasing, whatever the process.
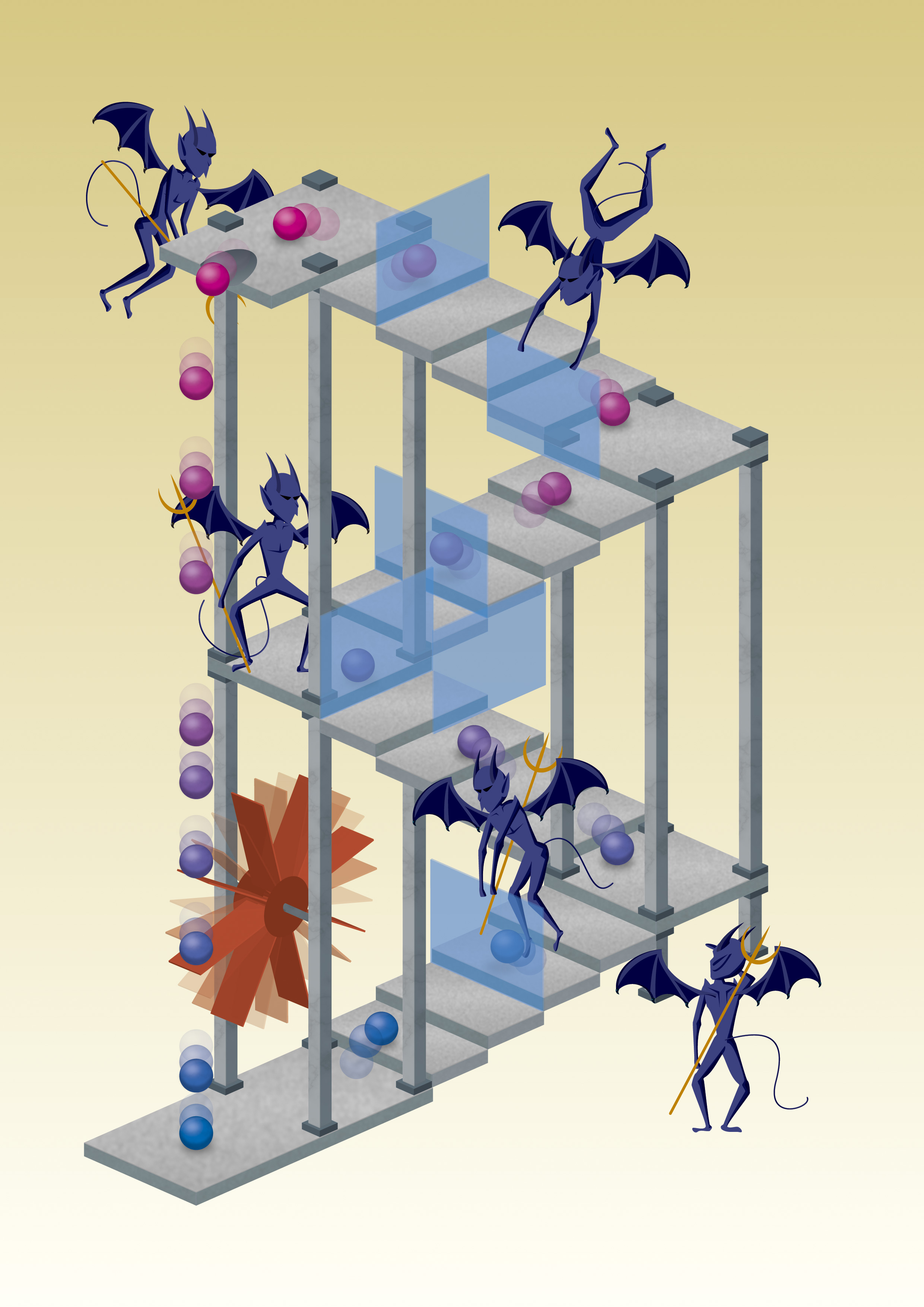
Maxwell's demon uses thermal motion of particles to let them move up a staircase and then blocks their way back. The effect is the same as with a box: particles on top of the stairs are warm, and cold at the bottom. Credit: Mabuchi Design Office / Yuki Akimoto
Well, asked James Clerk Maxwell back in 1867, what if you have a box filled with gas of a certain temperature. The box is separated into two compartments by a wall that has a small door. The door is controlled by a small ‘demon‘ that lets fast-moving gas molecules go into the right half, and leaves slow ones in the left. The left box would cool down, and the right one heats up. Overall, the box is more ordered than before. If the demon itself doesn’t use up any energy (which can be done), entropy would decrease, right? But according to the second law of thermodynamics energy is needed to create order, and the demon wouldn’t use any. Is this then a violation of the second law?
Well, actually not. The reason is that there is energy in information. To store a bit of information a system like a computer memory needs to be put into a defined state, either a ‘1’ or a ‘0’. This reduces entropy. And the same is true for the two compartments in a box. We use the information whether a molecule is fast or slow to separate them. The energy stored in that information is used to reduce the entropy of the system. So we are fine, the second law won’t be violated.
The energy that is contained in the information is tiny. For a single bit the lower limit is on the order of 10-21 joules. In comparison, one calorie, the old energy unit often used for food, corresponds to 4.184 joules.
In the more than 140 years since Maxwell came up with the hypothetical idea of the demon, a lot has happened technologically, and what he considered a thought experiment has already been done experimentally. However, none of these experiments have demonstrated this important aspect of the Maxwell demon: the conversion of information into energy, and the fact that if everything is considered the second law of thermodynamics still holds.
In a paper published in Nature Physics, researchers from Chuo University in Tokyo and the University of Tokyo report on an ingenious experiment that demonstrates exactly that. They take small polystyrene beads and let them float and rotate in an electric field. The electrostatic field that keeps the beads floating is comparable to a staircase. Every time the thermal movement of the beads make them go up a step, the demon (the applied electric field) blocks the way back and prevents them from falling down. Step by step the beads gain energy and climb the virtual staircase provided by the electric field.
Contrary to earlier, different Maxwell-demon experiments, here the experimental setup is sufficiently precise to allow the determination of how much information is converted into energy: about 28% of the energy stored in the information on the bead position goes into the system. Of course, this doesn’t consider all the energy put into the experimental apparatus, to keep the electric field going, etc. Despite that, even when only looking at the energy gained by the system through the information and assuming the demon uses zero energy, the researchers show that the second law of thermodynamics is not broken. The energy contained in the information is sufficient to compensate for the reduced entropy of the beads.
The laws of thermodynamics have withstood many attempts to prove them wrong, and this experiment once more confirms their validity. But what I find exciting here is the direct demonstration that information is converted into energy, and how that all fits so perfectly well into the thermodynamics picture. At university, thermodynamics is often treated as a true, but old and dusted theory that deals a lot with averaged quantities. But the matter of the fact is that thermodynamics remains a highly exciting ongoing research field that teaches us a lot about fundamental processes!
Reference:
Toyabe, S., Sagawa, T., Ueda, M., Muneyuki, E., & Sano, M. (2010). Experimental demonstration of information-to-energy conversion and validation of the generalized Jarzynski equality Nature Physics DOI: 10.1038/nphys1821
News and Views on the text:
Van den Broeck, C. (2010). Thermodynamics of information: Bits for less or more for bits? Nature Physics DOI: 10.1038/nphys1834
 This post was chosen as an Editor’s Selection for ResearchBlogging.org
This post was chosen as an Editor’s Selection for ResearchBlogging.org


Trackbacks/Pingbacks
[…] This post was mentioned on Twitter by ResearchBlogging.org, Paula Salgado and Hot In Science, Flipboard Science. Flipboard Science said: The demon is out of the bottle http://bit.ly/dyWNha […]
[…] The demon is out of the bottle. No, we haven’t regressed to a superstitious era of witches and demons — we’re talking about Maxwell’s demon, a hypothetical Second Law of Thermodynamics-violating creature! It is well-known now that this “demon” can’t violate the Second Law, but Joerg Heber at All That Matters describes an experiment that demonstrates explicitly the relationship between the demon and energy. […]
[…] The demon is out of the bottle « All that matters (tags: entropy physics energy information-physics information-theory) […]
[…] If you like to find out more about this experiment, take a look at my blog post. […]