
This USB flash drive used a bulk metallic glass for its casing. Credit: Liquidmetal Technologies.
This week I am attending the 2010 Materials Research Society Fall Meeting in Boston — one of the key meetings in materials science. One of the sessions is on bulk metallic glasses and their applications, which this year is a little special. It is organised in honour of the 50 year anniversary of the first demonstration of a metallic glass by William Klement, Ronald Willens and Pol Duwez from Caltech. Their paper on gold-silicon alloys was published in Nature on September 3rd, 1960. (In addition to Duwez and colleagues, David Turnbull must be mentioned here as one of the pioneers with several key contributions to the field. For example in 1948 he demonstrated that metals can be considerably undercooled below their crystallisation temperature.)
Metallic glasses have since become very interesting for applications that include sports products, coatings, power transformers where they reach several ten thousands of metric tons annual production, sports equipment, bioimplants and others. At the same time research researchers still try to learn more why these glasses form in the first place.
Why are metallic glasses so special?
All metals prefer to form crystals, as the metal atoms easily form structured bonds with other atoms. So easy in fact that even when the metal is melted some of that arrangement carries over into the liquid. This makes the formation of a crystal the preferred pathway once the melt is cooled down again. Glass on the other hand is amorphous, which means that the atoms are disordered and there is no long-range periodicity. This is not something metals prefer. Unlike the window glass made of silicon oxide. In comparison, metallic glasses are a very different animal.
So how to create a metallic glass? The answer is to give the metal atoms not the slightest chance to form a crystal. This means to cool the material superfast. Typical cooling speeds for the first metallic glasses made were on the order of a Million Kelvin per second: from about 1,300 degrees Celsius to room temperature in about a millisecond. This fast cooling can be done only for small samples, and the first metallic glass sample was a tiny flake: 0.2 mm2 area, and only 2o micrometers thick.
Then, about 15 to 20 years ago, William Johnson, Akihisa Inoue and others then discovered metallic glasses that are much easier to make, and with lower cooling rates. This meant that metallic glasses could now be produced in larger volumes, hence the name of these glasses: ‘bulk metallic glasses’. They have enabled the commercialization of metallic glasses.
The uses of metallic glasses
The absence of any long-range order in these metallic glasses comes as a benefit, not a curse. Metallic glasses might be disordered on the atomic scale, but this disorder looks very homogeneous when looking on a larger scale. The properties of crystals are easily messed up and degraded by imperfections in their structure. In contrast, in a metallic glass the imperfection is what makes the glass. There is no need to be concerned about degraded properties through disruptions.
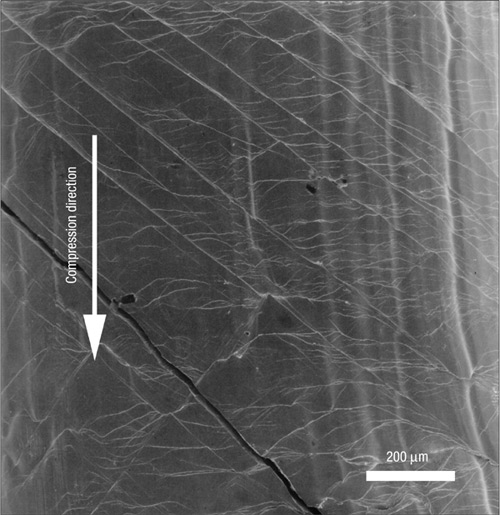
Close-up of a metallic glass after failure. The so-called shear bands are typical indicators of irreversible damage and eventually cause fracture. Image reprinted by permission from Macmillan Publishers Ltd: Nature Materials 2, 661 - 663 (2003).
An example of how useful this is are iron-based metallic glasses. They are excellent soft magnetic materials, which are magnetic compounds whose magnetisation follows that of an external magnetic field. In a crystal, imperfections can make it difficult for a soft magnetic material to change its magnetisation in response to an external field. This requires additional energy, and results in energy losses during the switching process. Not so in magnetic glasses, which are far more homogeneous and therefore show lower losses. Consequently, magnetic metallic glasses are used in transformer cores, where they are very economical in converting high voltages down to household levels. They are also great sensors for magnetic fields, for example as tiny compasses. In terms of looks, they look like regular metal.
Metallic glasses are also very tough, even though they can be very brittle once they fail. Still, their toughness means that they are very useful in a number of areas, ranging from medical instruments to possibly metal casings for electronic devices. Sports equipment is another, perhaps a more unusual one. But it highlights another intriguing property: as well as being tough, metallic glasses are also lightweight and elastic. Hence they bounce a ball better than steel. For this reason metallic glasses are used in ski equipment, baseball bats, golf clubs, and others.
Another very promising use are medical implants. Because they metallic glasses are so lightweight, tough and cheaper than high-quality steels or titanium alloys, they make very good implants. For example as prosthetic hip joints. Another application are degradable implants. MgZnCa metallic glasses are not only biocompatible, but also biodegradable. Mg, Zn, and Ca all occur naturally in the body and they can be absorbed by the body without harm. In the case of MgZnCa glasses, they are absorbed at a rate of about a microgram a day. This allows their use as nails to hold fractured bones together and that slowly dissolve as the bone grows. However, while successfully tested on animals, none of these bioimplants has been approved yet for human use.
Metallic glasses have not always been an easy system to study. It took more than 30 years after the original demonstration to come up with ways to fabricate them in sufficient quantities. Once that problem was solved, metallic glasses have slowly but surely made it into applications. Still, even after all that time a systematic and thorough understanding of the properties of metallic glasses is lacking. We are still far away from a situation where a metallic glass can be ‘designed’ according to specifications. But one thing is sure: metallic glasses will continue the successful path of the past 50 years. Cheers to that!
Reference:
KLEMENT, W., WILLENS, R., & DUWEZ, P. (1960). Non-crystalline Structure in Solidified Gold–Silicon Alloys Nature, 187 (4740), 869-870 DOI: 10.1038/187869b0
 This post was chosen as an Editor’s Selection for ResearchBlogging.org
This post was chosen as an Editor’s Selection for ResearchBlogging.org


Trackbacks/Pingbacks
[…] 50 years of metallic glasses. Under most natural conditions, metals form a nice crystalline structure. In a lab, however, they can be made to take a glassy state — Joerg Haber of All That Matters explains how it is done, and what it is good for! […]
[…] other anniversary, which was celebrated in September, is the first realization of a metallic glass. As I commented earlier, although metallic glasses haven’t reached the commercial significance of lasers , they are […]
[…] It is 50 years since metallic glasses have been discovered, and it is amazing how problems such as fabrication issues or low toughness have been overcome over the years. Most of you probably have never held a metallic glass in your hands. But with advances like that, metallic glasses may soon become much more widespread. […]