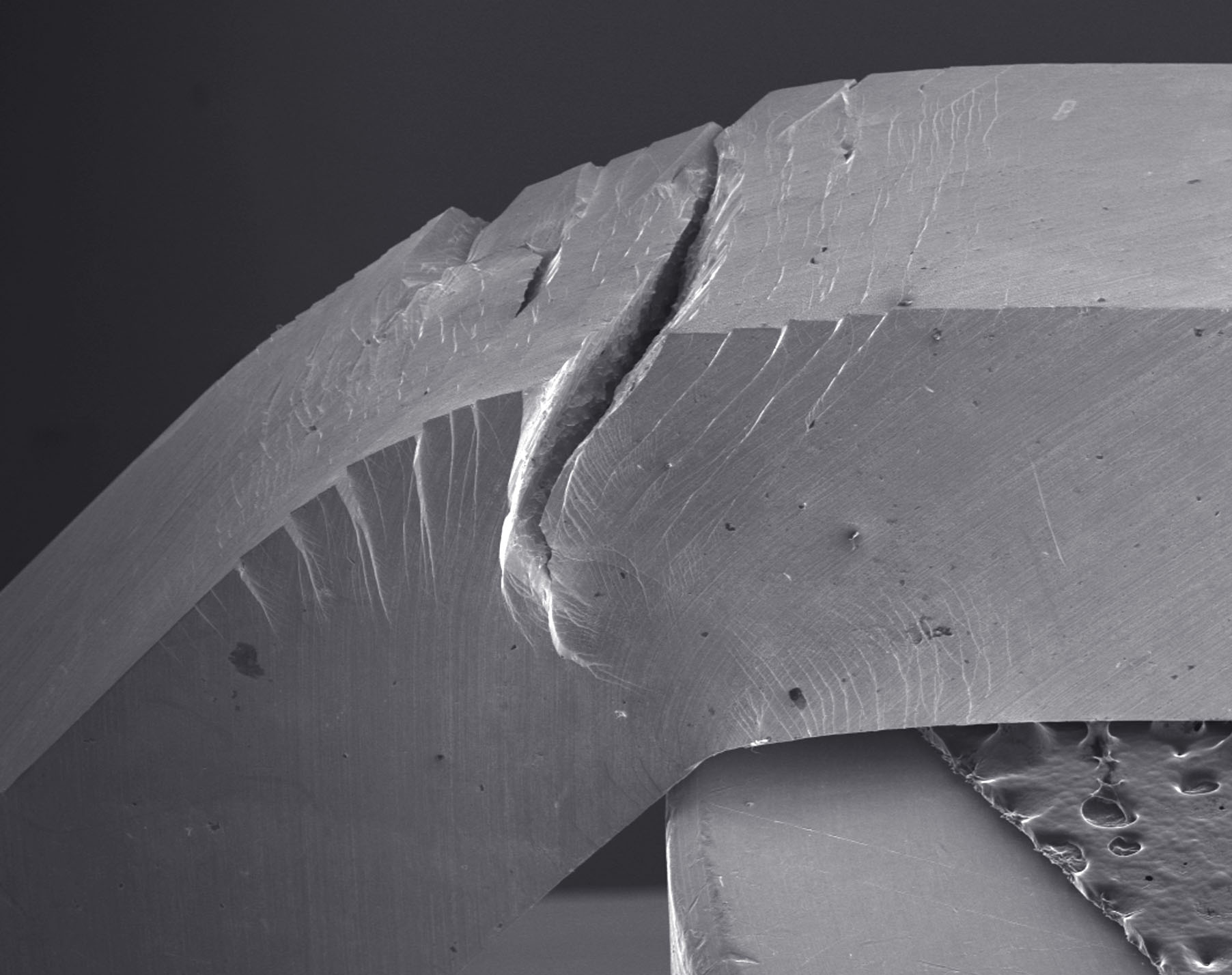
A tough metallic glass. This Pd79Ag3.5P6Si9.5Ge2 glass did not fracture catastrophically even after undergoing significant bending. Credit: Maximilien E. Launey
Glasses are very useful structural materials, because they are strong. You can stand on a glass table without it breaking. But once you put too much stress on a glass it breaks. Steels on the other hand are much tougher — which means that they show a much stronger resistance to fracture: rather than suddenly breaking under stress, steels tend to deform first. This toughness of steel is one of the obvious reasons why steel skeletons instead of glasses are used for the construction of large buildings.
This conception of glasses not being very tough is now made history by Marios Demetriou and colleagues from the California Institute of Technology and The University of California, Berkeley. They have discovered a glass that is not only strong, but also tough as steel. It is a metallic glass, which means that it dominantly consists of metal atoms. Like other metallic glasses it isn’t transparent, but looks like a normal metal — except that unlike crystalline metals its atomic structure is more or less random. The damage tolerance of this metallic glass, its combination of strength and toughness, is higher than any known material. “It has been usual to regard metallic glasses as damage sensitive, but over recent years it has become increasingly recognised that they can be tough. This new paper marks a further decisive shift in showing that metallic glasses can be very tough indeed,” says Lindsay Greer from Cambridge University, who studies the properties of metallic glasses.
This discovery of the most damage resistant material known so far is all the more remarkable as strength and toughness are often considered as mutually exclusive. It seems even more surprising to find such a property in a glass. One of the problems in developing metallic glasses with superior properties is to find combinations of elements that actually form a glass and whose atoms do not crystallize into regular metal alloys. Avoiding crystallization can be quite difficult and finding the right combination of metals that form a glass is often based on trial and error.
The metallic glass that Demetriou and colleagues have discovered has the formula Pd79Ag3.5P6Si9.5Ge2. Guided by a few principles, its discovery was to some degree serendipitous, and started with a palladium-based glass containing small portions of phosphorus, silicon and germanium. This glass turned out to be rather tough already, which spurred further research. The researchers eventually added silver, which considerably enhanced the toughness to its present record-breaking value. “The toughness was recognized to be indeed remarkable when heavy-duty cutting jaws were seen to fully sink into bulk samples by plastically deforming the glass without breaking it using very high force,” recounts Demetriou. The glass deformed, but didn’t break.
With such great properties, what can we expect from this glass? Certainly, we won’t see steel being replaced by metallic glasses soon. “Applications must be very limited by the high price of the main noble-metal constituent,” comments Greer on the large amount of palladium in the glass. Furthermore, while the glass is tough to bend, its weakness appears when it is pulled from both ends. Then it doesn’t stretch as steel does to some degree, but like pretty much all other metallic glasses it breaks easily.
Still, owing to their high strength metallic glasses have found a number of interesting applications, for example as coatings. The toughness of this new metallic glass will make them even more attractive for that. Metallic glasses can also be quite corrosion resistant. Combined with their strength – and now also toughness – this makes them ideal candidates for bioimplants such as hip replacements. Demetriou sees another potential application: “Many noble-metal alloys, including palladium, are currently used in dentistry due to their chemical inertness and resistance to oxidation, tarnish and corrosion. Owing to its superior damage-tolerance capacity, the present palladium alloy can be thought of as a superior alternative to conventional palladium dental alloys. […] The absence of any elements considered toxic or allergenic from the composition of the present glass will likely promote good biological compatibility.”
It is 50 years since metallic glasses have been discovered, and it is amazing how problems such as fabrication issues or low toughness have been overcome over the years. Most of you probably have never held a metallic glass in your hands. But with advances like that, metallic glasses may soon become much more widespread.
Reference:
Demetriou, M., Launey, M., Garrett, G., Schramm, J., Hofmann, D., Johnson, W., & Ritchie, R. (2011). A damage-tolerant glass Nature Materials DOI: 10.1038/nmat2930
Note: While I made every attempt to present these results in an objective manner, please be aware that I was the handling editor for this paper. For more details see the disclaimer.


January 10, 2011 at 04:46
With >75% Pd w/w, to a common man, it sounds more like a Pd alloy than a “glass”.
With so much of Pd, I think it must be denser than a “glass” also.
January 10, 2011 at 10:25
What makes it a glass is not the composition – you can have glasses made from a single element. It is the atomic structure. If the atoms are disordered it is a glass. If they are ordered throughout, it is a crystal. Take a combination of metals such as the present one and melt it – whether it becomes a glass or an alloy depends how fast you cool it down again. If you cool it slowly, it becomes a crystal. If you cool it fast, the atoms don’t have time to arrange into a crystal structure but are disordered – then you have a glass. But note, not all elements or compositions form glasses, it is a game of (educated) trial and error….
January 10, 2011 at 04:46
Rather, denser than steel also!
January 10, 2011 at 10:26
– possibly. But note that metallic glasses exist that have a very high iron content, like steel. But they haven’t proven as tough yet.
September 6, 2011 at 11:55
how is this glass produced?
how about mechanical alloying(MA) in metallic glass preparation?is it (MA) a reliable method?
what are the limitations?
September 6, 2011 at 12:03
Metallic glasses mostly are produced by casting from the melt. Key here is that in order to avoid crystallization of the melt and to produce a glass the temperature of the melt has to drop extremely fast. Hence, mechanical alloying cannot be used.
April 27, 2013 at 19:26
Do you know if there is anyone manufacturing this material yet? I have some items that would greatly benefit by being made of this material