Hydrogen sensing at the nanoscale. Hydrogen molecules (red) are absorbed by a palladium nanoparticle (silver) and the resulting changes in optical properties amplified by a gold antenna. (c) Mario Hentschel, Na Liu, Harald Giessen
Sensing the presence of molecules in gases and liquids is a billion dollar business. Just think about all the carbon monoxide detectors in private homes, or blood glucose sensors. In particular for many technical and scientific applications, ultrasmall and precise sensors are desired. This includes sensors to measure gases in catalytic nanoreactors and fuel cells, or the monitoring of biochemical processes.
Laura Na Liu and Ming Tang from the group of Paul Alivisatos, director of Lawrence Berkeley Lab in the USA, and Mario Hentschel from Harald Giessen‘s group at the University of Stuttgart in Germany have now developed a new class of optical nanoscale sensors that are able to measure specific molecular concentrations down to single particles. This, says Alivisatos, “should pave the road for the optical observation of chemical reactions and catalytic activities in nanoreactors, and for local biosensing.” Their paper is published this week in Nature Materials (declaration of interest: I was the handling editor of this paper, although I like to stress that I don’t benefit in my day job by blogging about this work).
To pick up the presence of only a handful of molecules requires a strong amplification able to detect the tiniest changes in sensor properties. Plasmonic effects in metal nanostructures are ideal for this. Light at the plasmon frequency excites electronic motions at the surface of metals that act like antennas and create very strong local optical fields. The smaller and pointier these nanostructures, the larger the local concentration of light fields. This is a well-known effect that can be used to enhance the performance of solar cells. Such schemes can also be used for sensing, although often plasmon sensors have issues either with selectivity to a target molecule, sensitivity, or with reliability.
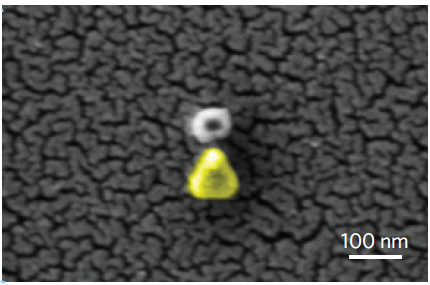
Scanning Electron Microscope image of the plasmonic antenna. Reprinted by permission from Macmillan Publishers Ltd. Nature Materials, advance online publication (2011)
Here, to achieve a reliable sensitivity to hydrogen, a palladium nanoparticle is placed close to the tip of a tiny gold triangle, at distances as small as 10 nanometers. Palladium efficiently absorbs hydrogen, and this changes its optical properties. These changes in the palladium optical properties are strongly amplified by the gold nanoantenna, and appear as a wavelength shift in the structure’s plasmonic resonance. For example, the presence of 2% hydrogen in a neutral nitrogen gas shifts the wavelength by up to 9 nanometers, which can be easily detected.
Of course, with palladium being a metal, shifts would appear in the plasmon resonance of the nanoparticle as well, but for palladium these resonances are considerably broader than for gold, which reduces sensitivity. The key advance here is to separately optimize the two steps involved in the sensing. The palladium is chosen for its high sensitivity to hydrogen, whereas the nearby gold nanoantenna ensures a strong plasmon response.
Another key advantage is that only a single device is needed in the experiment. So far, plasmonic sensing devices often average over a larger number of structures, which inevitably means that the system’s response is averaged and therefore less sensitive. Here, tiny gas volumes can be measured by a single device. On this small scale a 2% hydrogen concentration in the environment corresponds to only a few hydrogen atoms on the palladium nanoparticle.
Another key advantage is the versatility of the approach. The palladium disk could be replaced with almost any material or composite that can be fabricated next to the gold antenna. That means a large variety of compounds can potentially be detected. And they don’t necessarily have to be gases, says Liu. “Our antenna-enhanced plasmonic sensing technique comprises a noninvasive scheme that is biocompatible and can be used in aqueous environments, making it applicable to a variety of physical and biochemical materials.” Without doubt, success is in the air…
Reference:
Liu, N., Tang, M., Hentschel, M., Giessen, H., & Alivisatos, A. (2011). Nanoantenna-enhanced gas sensing in a single tailored nanofocus Nature Materials DOI: 10.1038/nmat3029


Trackbacks/Pingbacks
[…] This image is from a really interesting paper in Nature Materials from the Alivisatos group at Berkeley. A great description of the research can be found here. […]