Understanding the properties of something chaotic such as a bowl of spaghetti may seem a daunting task. But that’s what Garry Rumbles from the National Renewable Energy Laboratory in the USA, Natalie Stingelin from Imperial College London in the UK, and coworkers are trying to do. With success. They study polymers – long spaghetti-like molecules made of repeating atomic subunits – and have now uncovered how the microstructure of these polymers controls the behaviour of optically generated electrical charges in such a tangled molecular web, with important implications for the design of electronic devices.
The physical properties of polymers depend a lot on the length of the molecules as a whole, the atomic make-up of their structural units and the physical interactions between the individual strings. That’s why polymers come in so many forms, from hard plastics to stretchable synthetic rubbers. And what Stingelin and Rumbles now show is that also their electronic properties depend not only the chemical make-up of the polymers, but also the details of their structure and their molecular weight. This has dramatic consequences for the search of new polymers for various optical and electronic applications, says Stingelin. “Are there otherwise wonderful polymers out there that were cast aside because their creators tested the wrong molecular weight? We think it’s quite possible.”
The implications of their findings on the dynamics of optically generated electrical charges in different polymer structures could be particularly relevant for the development of better polymer solar cells, says Michael McGehee from Stanford University. “Several of the concepts in this paper are going to lead to a much better understanding of how polymer solar cells work. I think that polymer solar cells are nowhere near as well understood as many people think. Most models ignore the fact that there are both crystalline and amorphous regions in the film.”
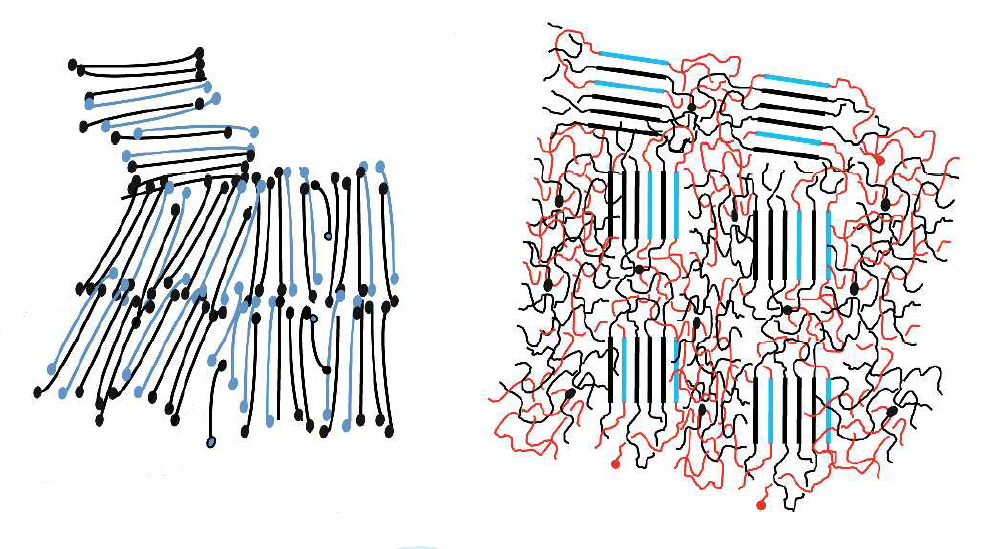
Even if their structural units are the same, polymers with different lengths have different microstructures. Films with low molecular weight align into crystalline regions, whereas molecules of higher weight form a mix of crystalline and amorphous regions. (c) 2011 Wiley Periodicals, Inc. Journal of Polymer Science Part B: Polymer Physics. Used with permission.
The coexistence of these crystalline and amorphous regions is one of interesting properties of polymers. Whereas the shorter, low-weight molecules tend to arrange in ordered, crystalline sections, the longer and heavier molecules consist of both, crystalline parts and disordered, amorphous sections (see figure).
The fraction of ordered to disordered domains depends strongly on the length of the polymers, their molecular weight. Rumbles and Stingelin have studied films of the same polymer, poly(3-hexylthiophene) – P3HT – but for samples having molecules with different weight. “We have collected over the period of more than five years various materials from different groups and suppliers, which really allowed us to perform such an in-depth study,” says Stingelin.
Provided with such a unique collection of different samples of the same polymer, the researchers undertook a comprehensive study into the properties of these films, such as the efficiency of the conversion of light to electrical charges, how long these excited charges remain in the films, and how mobile they are. An obvious finding from these experiments is that electrical charges live longer in the low-weight molecules, which have more crystalline regions, than for the heavier molecules that have more amorphous ones. There are simply too many possibilities for electrons to lose their energy in the disordered parts.
The data also yielded a surprise finding. One of the key parameters for solar cell operation is the number of charge carriers generated by light, multiplied by their mobility. This combined figure of merit basically describes how efficient a material is not only to convert light into electrical charges, but also to extract these charges out of the solar device. And here, the polymers with high molecular weight are much better than the lighter ones. This seems counterintuitive at first, because disordered materials are usually worse for solar cells.
The reason why overall the heavier polymers are better is that the interface between the crystalline and amorphous regions seems very efficient in separating the negatively charged electrons and their positive counterparts, the holes from each other. The holes go into the crystalline part, and the electrons stay in the amorphous one, which makes it easier to extract them out of the device.
But this does not necessarily suggest that the heavier, more disordered molecules are better, warns Stingelin. “We should be clear that the charge separation mechanism we demonstrate in our paper is not likely to lead to efficient solar cells by itself […] disorder can be, and usually is bad for organic solar cells.” Rather, the study suggests that it is the interfaces between amorphous and crystalline regions that are important, because there the positive and negative charges are separated.
Therefore, in future it will not only be relevant to simply optimize the weight of the molecules, meaning the mix of crystalline and amorphous regions, but also their structural arrangement and the interface between the different regions, says McGehee. “I think that we will have much more detailed and accurate models over the next couple years and hope that these models will ultimately result in solar cells with higher power conversion efficiency.”
Reference:
Reid, O., Malik, J., Latini, G., Dayal, S., Kopidakis, N., Silva, C., Stingelin, N., & Rumbles, G. (2011). The influence of solid-state microstructure on the origin and yield of long-lived photogenerated charge in neat semiconducting polymers Journal of Polymer Science Part B: Polymer Physics DOI: 10.1002/polb.22379


October 18, 2011 at 23:35
Charge separation and extraction for polymeric cells seem to follow the same principle for DSSC. Generating and electron-hole pair doesn’t mean to be able to extract electron therefore having a functional solar cell.