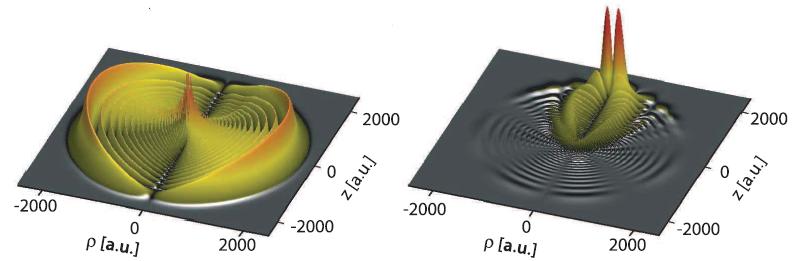
Permanent electric polarization. Left: the electron density of the rubidium Rydberg electron in one of the atoms of the molecule. The asymmetry is hardly visible. Right: The same electron density, but with the density of the Rydberg atom on its own subtracted. The difference clearly shows an asymmetric distribution of electrons roughly at the position of the second rubidium atom, causing an electric polarization. (c) 2011 Science Magazine
Apply an electric field to a material, and its positive and negative charges will separate, creating an electric polarization. This is the fundamental effect behind capacitors used in electronics as well as in ferroelectrics used in some computer memories. In the latter case, to achieve a permanent electric polarization, the positive and negative charges need to be shifted permanently. This is the case if in a crystal all positive and all negative ions in a crystal are shifted in the same way with respect to each other.
The separation of positive and negative ions in a crystal can lead to a permanent electric polarization. A similar effect has now been achieved with electrons.
In a paper in Science from last week, researchers from the group of Tilman Pfau at the University of Stuttgart in Germany with colleagues from a number of other institutions have now demonstrated an entirely new way of achieving permanent electric polarization – namely by using electrons and not ions. This effect is remarkable, because electrons usually are much more mobile than atoms. Normally, any charge imbalance in the electron distribution of a material or molecule is easily neutralized simply by shifting electrons in the molecule around. At the same time, looking far ahead, such electron-based effects could lead to applications where the electric polarization needs to switch ultrafast.
However, the molecule studied by the researchers is quite different to usual molecules. It is formed by two rubidium atoms, which means that normally it should not show any electric polarization, simply because both atoms in the molecule are identical and for symmetry reasons no positive or negative ions would form in the first place.
But although they are both rubidium atoms, here there is a crucial difference in the electronic states. One of the atoms is in its energetic ground state, while the other is a so-called Rydberg atom, which means that its outermost electron is excited into a very high energy state and circulates the atom’s nucleus at a large distance. Rydberg atoms are huge in comparison. Here, the rubidium atom is roughly about 50 nanometres in size, corresponding to about 1,000 times the size of a oxygen molecule – and is larger also than the transistors in modern computer chips.
Obviously, for molecules made of an atom in its ground state and one in one of these Rydberg states there is a large imbalance in the electronic states. But still, how is it possible that in such a molecule any electric polarization forms? With one electron being that far out, the interaction between the electrons of the two atoms is extremely small. The reason a polarization forms in the molecule is that the electronic state of the Rydberg atom extends so far out that it extends across the other rubidium atom. The presence of this atom alters the Rydberg atom’s electronic states at the location of the regular atom. That way, the symmetry of the electronic states of the molecule is broken, and this imbalance is the origin of the permanent electric polarization. To detect such a small imbalance, the researchers measured the shift of the molecule’s optical resonances in an electric field, which clearly confirms the effect.
More generally, electron-based polarizations could be very interesting because the electrons of a molecule are much more mobile than atoms themselves, so that the switching of the polarization – for example turning it around by 180 degrees – should occur much faster than in conventional materials, where the polarization is based on ions. This could be interesting for applications such as ultrafast data storage, where up and down of the polarization would represent the 1 and 0 of a computer bit.
Of course, we are still far away from such dreams. The Rydberg atoms are stable only at ultralow temperatures a few millionth of a degree above absolute zero. And also, here this effect seems again down to atomic positions, even if somewhat indirectly; it is the position of the second atom that determines the electric polarization. On the other hand, there are theoretical predictions where some molecules could also show electron-based electric polarization effects at much higher temperatures. Therefore, it seems to me this study is only the beginning for electron-based polarization effects, and we should look forward to similar observations being made in other molecules, too.
Reference:
Li, W., Pohl, T., Rost, J., Rittenhouse, S., Sadeghpour, H., Nipper, J., Butscher, B., Balewski, J., Bendkowsky, V., Low, R., & Pfau, T. (2011). A Homonuclear Molecule with a Permanent Electric Dipole Moment Science, 334 (6059), 1110-1114 DOI: 10.1126/science.1211255


Trackbacks/Pingbacks
[…] Electrons out of balance Apply an electric field to a material, and its positive and negative charges will separate, creating an electric polarization. This is the fundamental effect behind capacitors used in electronics a… Source: blog.joerg.heber.name […]