Move over graphene, there is competition in town. A new type of two-dimensional materials – with the far less appealing family name, transition metal dichalcogenides – are increasingly gaining attention. Well, at least they’re giving it a shot. Graphene, a sheet of carbon atoms only one atomic layer thick, still has plenty going for itself in terms of electronic, optical and mechanical properties. There seems nothing that graphene can’t do.
On the other hand, there are also limits. When it comes to its electronic properties graphene is not a semiconductor in the same was as silicon is. It is lacking a bandgap, a gap in its electronic states that is important for light emitters and for some electronic devices.
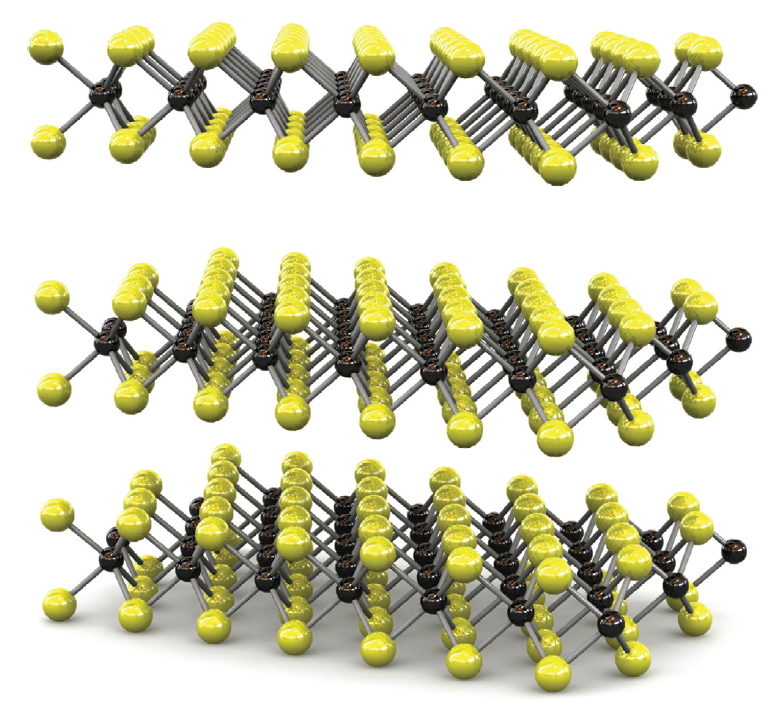
Schematic model of transition metal dichalcogenide atomic layers. The yellow balls represent the chalcogenide atoms, the blue ones the transition metals. Reprinted by permission from Macmillan Publishers Ltd. Nature Nanotechnology (2012). doi:10.1038/nnano.2012.193
Transition metal dichalcogenides offer an advantage there. They are semiconductors, and they can have a bandgap. And as their name says, they are formed by a combination of chalcogens such as sulphur or selenium and transition metals such as molybdenum or tungsten. Typical examples are MoS2 or MoSe2. These materials have become such hot stuff now that their properties have been reviewed in this month’s issue of Nature Nanotechnology. And even though the field is still young, there is plenty to review.
For graphene, there is a difference in properties to its bulk counterpart, graphite The same is also true to some degree for these new two-dimensional materials. MoS2 for example only has an indirect bandgap in bulk form, which is unsuitable for light emission. But for single layers the bandgap increases and becomes direct, and the material is of interest for photonic applications. And in electronics, even field effect transistors have been realized from the material. They work at room temperature, and at decent speeds.
There are of course still a lot of problems to solve, not unlike those known also from the early days of graphene. Fabricating these compounds in large quantities, reducing the number of impurities, or adding the right kind of impurities to achieve the right polarity for electronic applications, all this is still very difficult. But if graphene does indeed make for a good comparison, such fabrication issues will be overcome eventually. And at that point these compounds then can be really put to the test. There is still so much that we don’t yet know about these single layer sheets, about their intrinsic properties.
Overall, I am a bit doubtful whether they will be an compelling all-round materials as graphene is, but one thing is clear based on all the buzz going on in the field: we will see a lot more of these materials in the near future.
Reference:
Wang, Q., Kalantar-Zadeh, K., Kis, A., Coleman, J., & Strano, M. (2012). Electronics and optoelectronics of two-dimensional transition metal dichalcogenides Nature Nanotechnology, 7 (11), 699-712 DOI: 10.1038/nnano.2012.193


November 13, 2012 at 22:12
Reblogged this on trackingthefuture and commented:
Add your thoughts here… (optional)