The past year has been a great year for science with major advances in several areas. Too many exciting results to mention here. Instead, to reflect about the past year I have chosen a representative paper for each month of the year that I hope can serve as an example of the great science going on in a number of research fields. Of course, this is a highly subjective and personal collection, and indeed there might be others worth mentioning. But the aim was also to provide a balanced overview of the year that covers a variety of topics.
Of course, if you have an exciting paper to add, please feel free to use the comments section below to let us know!
Anyway, enough said, here are some of my highlights from the past year:
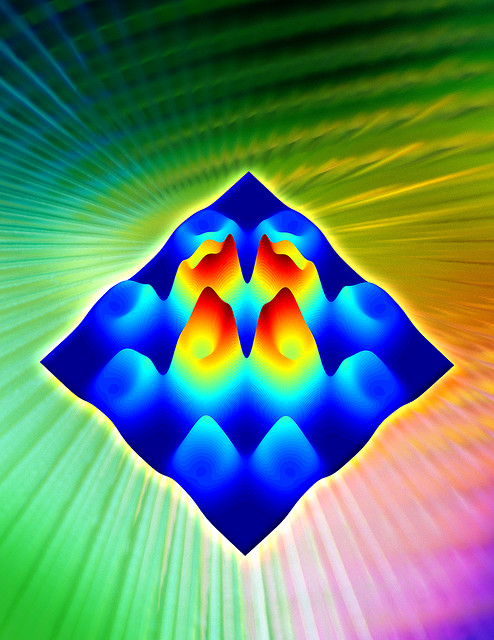
Simulations of electronic excitations in an iron-based superconductor. Image by Oak Ridge National Laboratory via flickr.
JANUARY – iron-based superconductors
Since they were discovered in 2008, iron-based superconductors, the pnictides, have been one of the hottest topics in condensed matter physics. Part of their appeal stems from the fact that they are based on iron, which is a magnetic element. Normally, magnets and superconductivity exclude each other.
The iron-based compounds have a similar crystal structure as the so-called cuprates, which are the materials with the highest superconducting temperatures known. The mechanism for these high-temperature superconductors is unknown, and studying the iron-based superconductors may also be relevant to the understanding of the cuprates.
This paper published in Science shows for the first time that the electrons in the iron-based superconductors show a periodic arrangement that is different to the periodicity of the atoms in the crystal. Similar observations have been made in the cuprates, and their understanding is considered important to the mechanism of high-temperature superconductivity.
Chuang, T., Allan, M., Lee, J., Xie, Y., Ni, N., Bud’ko, S., Boebinger, G., Canfield, P., & Davis, J. (2010). Nematic Electronic Structure in the “Parent” State of the Iron-Based Superconductor Ca(Fe1-xCox)2As2 Science, 327 (5962), 181-184 DOI: 10.1126/science.1181083




December 27, 2010
3 Comments