This week my colleagues at Nature Chemistry landed an impressive scoop, the publication of a paper by Michael Strano and colleagues from MIT on self-regenerating solar cells.
The performance of any kind of solar cell tends to degrade over time. This is particularly the case for organic solar cells, where sunlight can easily destroy the structure of the molecules used. Natural light-harvesting processes have a similar problem, for example during photosynthesis. The way plants solve this problem is through a self-repair mechanism.
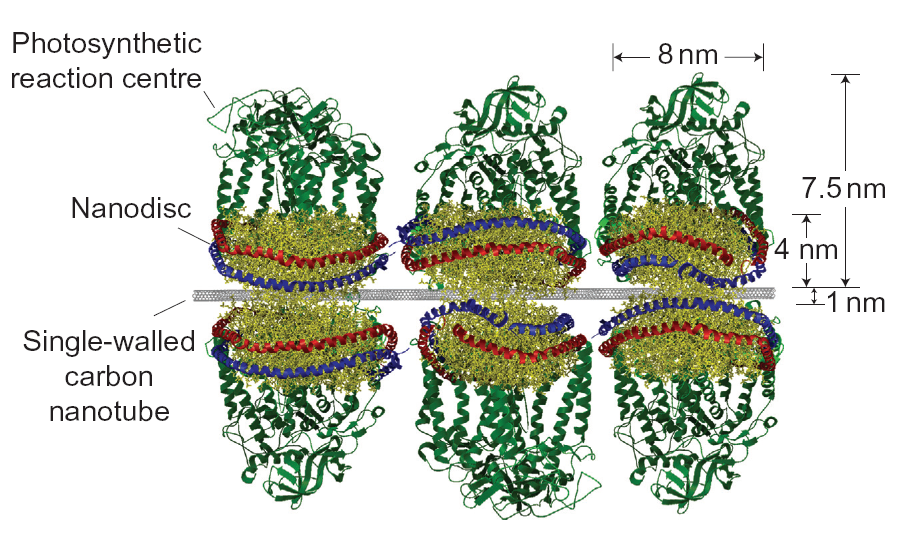
Schematic of the regenerating solar cell consisting of light-absorbing proteins, lipid disks and carbon nanotubes. Reprinted by permission from Macmillan Publishers Ltd. Nature Chemistry, advance online publication (2010)
Taking cues from such self-regeneration strategies, Strano and colleagues use a concept that is surprisingly simple. They prepare a solution containing carbon nanotubes, bacterial light-harvesting proteins and discs made from lipid molecules — the structural components that form the membrane of cells. Once the surfactant that keeps all these molecules separate is removed the molecules assemble themselves: the proteins bind to the lipids, which then attach to the carbon nanotubes. No assembly required.
During solar cell operation sunlight is absorbed by the proteins and creates electronic charges that are transported along the carbon nanotubes to the electrical contacts of the solar cell. To regenerate the proteins damaged by the sunlight, surfactant is added again, along with a small quantity of new proteins to replace damaged molecules. This dissolves the structure. But once the surfactant is removed the molecules reassemble, fully repaired.
In the study, the solar cells ran on a 40 hour cycle: 32 hours of operation, followed by 8 hours regeneration. Despite so many hours of regeneration overall cell performance was up by a remarkable 300% in comparison to cells that are not regenerated. And this could be just the beginning. At the moment, as soon as the cells are turned on they lose about 60% efficiency within the first hour or so of operation. Delaying the onset of degradation or finding a more efficient way of regeneration should lead to further enhancements. But who knows, nature may have a solution to this problem, too.
Reference:
Ham, M., Choi, J., Boghossian, A., Jeng, E., Graff, R., Heller, D., Chang, A., Mattis, A., Bayburt, T., Grinkova, Y., Zeiger, A., Van Vliet, K., Hobbie, E., Sligar, S., Wraight, C., & Strano, M. (2010). Photoelectrochemical complexes for solar energy conversion that chemically and autonomously regenerate Nature Chemistry DOI: 10.1038/NCHEM.822



September 10, 2010
1 Comment