
A coaxial cable plug. The coaxial nanolaser is more than 15,000 times smaller. Photo by mikemol via flickr.
When Oliver Heaviside invented the coaxial cable in 1880 he could not have foreseen the implications of his idea on modern nanotechnology. His coaxial cables consist of three layers: an inner metallic core, surrounded by an insulator, surrounded by a metallic layer on the outside. The benefit of this design is that the outer metallic layer shields the electrical signal through the cable from outside interference. This makes coaxial cables very useful for information transfer, and coax cables are used for TV antenna cables or some computer network cables. Mercedeh Khajavikhan, Yeshaiahu Fainman and colleagues from the University of California, San Diego now present a completely new application: they have fabricated coaxial lasers on the nanoscale that turn on without the usual minimum threshold power of usual lasers. To do this they had to shrink the coaxial cables first. These lasers are more than 15,000 times smaller than typical coaxial cables.
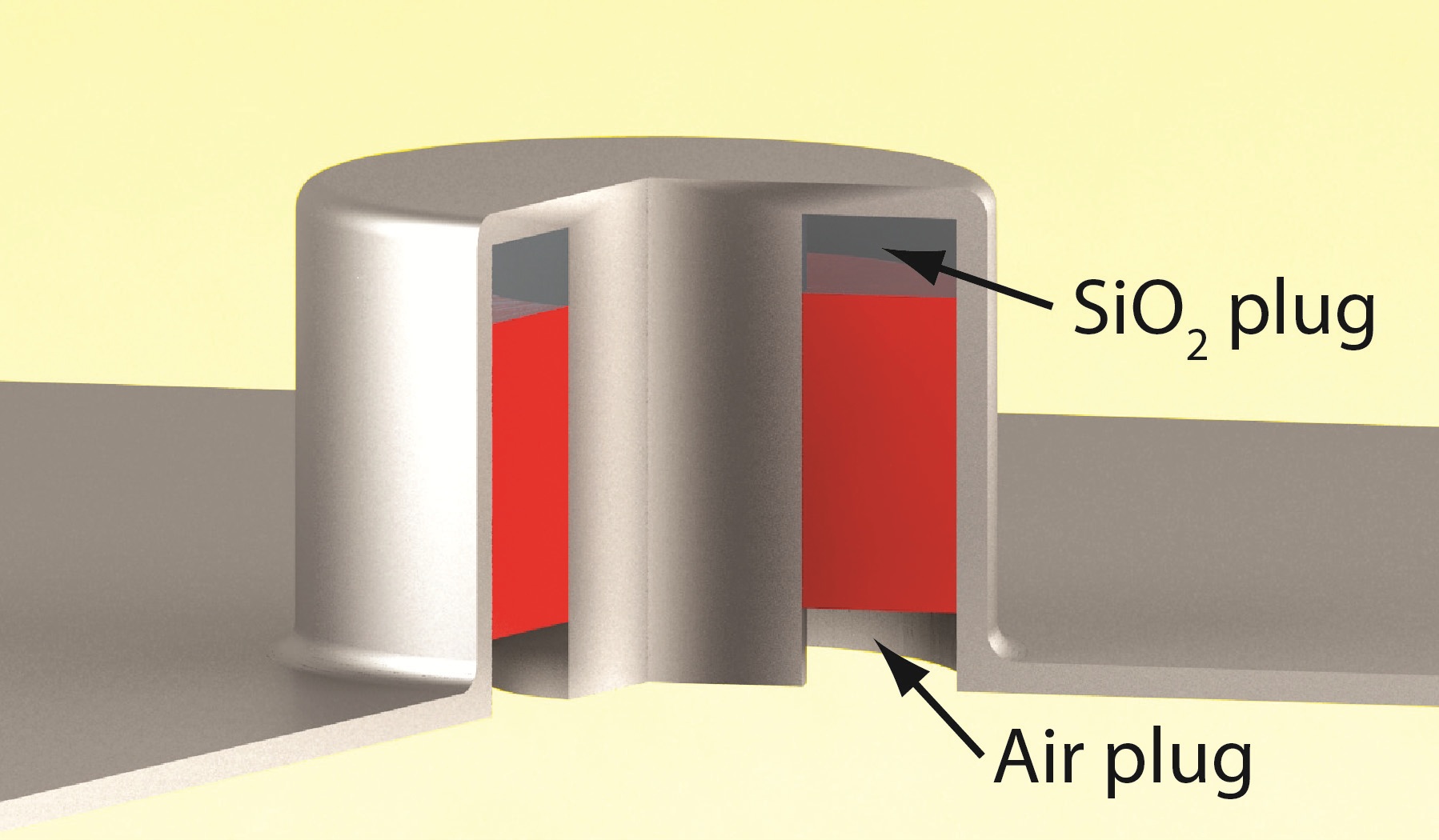
The nanoscale coaxial laser. Similar to coaxial cables it consists of an inner metal pillar and an outer metal shield. The structure is also protected from interference from the top. Inside is a semiconductor light emitter (red; insulated from the top metal through a SiO2 plug). The laser light exits through the hole in the substrate. Figure by Mercedeh Khajavikhan and Aleksandar Simic.
The benefit of a coaxial cable is that between the core and the outer metal layer well-defined and controlled electromagnetic waves can propagate shielded from any outside influence. Furthermore, shrinking such a device to the nanoscale – to length scales comparable to the light used – means that only the smallest optical beam pattern for the wavelength of light, known as the fundamental mode, fits into the small space between the metal structures. The other modes would be too large. Continue reading…


February 8, 2012
2 Comments