Today it was announced that the 2010 Nobel prize in physics goes to Andre Geim and Konstantin Novoselov “for groundbreaking experiments regarding the two-dimensional material graphene.”
Geim’s and Novoselov’s work on graphene has been frequently predicted for the Nobel prize, although interestingly graphene has been studied long before they entered the field. Studies on graphene go back at least to the 1970s, and the name for this atomically thin layer of carbon came into more wider use in the 1980s.
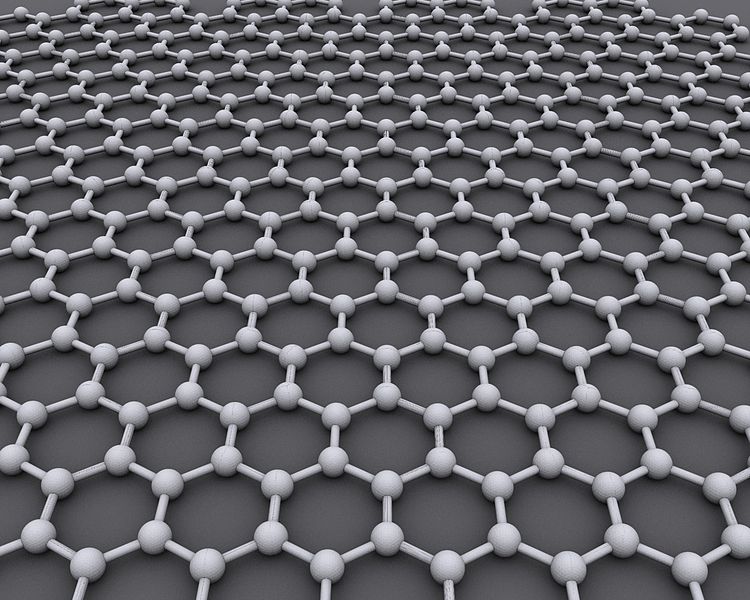
A model of graphene. Image by AlexanderAlUS via Wikimedia Commons.
So what is the big deal with Geim’s and Novoselov’s research? Well, they developed a really simple method to fabricate graphene. Graphene is a close relative of graphite. Graphite consists of layers of carbon where in each layer the carbon atoms arrange as hexagons. These layers can be visualized as sheets of chicken wire.
Graphene is nothing but a single one of those sheets that make up graphite. The method Geim and Novoselov developed in 2004 to extract graphene is stunningly simple. Take a graphite pencil and write with it on a piece of paper. Then take a post-it note and use it to lift off tiny pieces of graphite. Look under the microscope and identify the single layer ones, and that’s it! But of course, in the meantime more efficient fabrication technologies for graphene have been developed.
As Geim, Novoselov, and many others consequently demonstrated, graphene is a unique material, fundamentally different to graphite. It is highly conducting, and electrons can travel through it for long distances without being deflected. This makes it interesting for fast transistors, and this is the point also of Geim and Novoselov’s ground-breaking first paper on graphene published in Science in 2004. Graphene shows also some interesting electronic properties owing to its electronic band structure, even the fractional quantum Hall effect.
And then of course the electronic bonds in graphene are very strong, which not unlike carbon nanotubes makes it an excellent structural material. Then there are possible applications in molecular sensing and many others. All this makes graphene highly interesting for researchers from many scientific areas. However, some of the rationale expressed by the Nobel Committee strikes me a bit odd, evidenced by this tweet: “According to Nobel Committee, practical applications for graphene include touch screens, fast transistors & DNA sequencing. #nobelprize.”
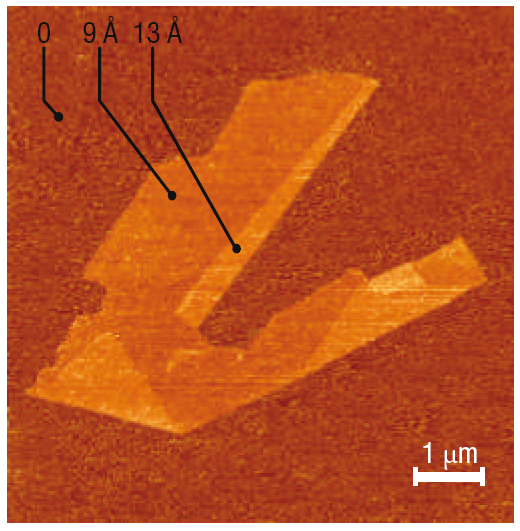
Flakes of graphene. Reprinted by permission from Macmillan Publishers Ltd. Nature Materials 6, 183-191 (2007).
Indeed, I agree that graphene has potential in all these areas. But we still have to see those promised applications. The last application in this list, DNA sequencing, is from a Nature paper less than a month old!
As for transistors, well, the edges of graphene cause a lot of problem, and so does fabrication. I recently blogged about attempts to use nanowires to make graphene transistors, which are still very far off commercial uses as well. And when it comes to the band structure properties of graphene such as the so-called Dirac point, well, topological insulators show similar physics but could be far more promising.
Graphene is a highly interesting and versatile material with cool properties. But when it comes to applications, well, we will see whether an all-rounder such as graphene will be able to beat incumbents. This is certainly far from clear yet. So please let’s stay realistic on the practical implications of graphene.
Overall of course, I am very happy for Geim and Novoselov, they certainly deserve the prize. At the same time I find it interesting that Sumio Iijima‘s discovery of carbon nanotubes hasn’t been rewarded yet.
In any case, it is a great week for UK science, with Nobel prizes in medicine and physics going to UK institutions. This recognition shows the high standard of UK science, which is presently in severe danger from government budget cuts.
Reference:
Novoselov, K., & Geim, A. (2004). Electric Field Effect in Atomically Thin Carbon Films Science, 306 (5696), 666-669 DOI: 10.1126/science.1102896
Further reading:
Geim, A., & Novoselov, K. (2007). The rise of graphene Nature Materials, 6 (3), 183-191 DOI: 10.1038/nmat1849
 This post was chosen as an Editor’s Selection for ResearchBlogging.org
This post was chosen as an Editor’s Selection for ResearchBlogging.org


October 5, 2010
29 Comments