Light is special. In our everyday experience it behaves like a wave, which gets reflected, refracted and shows interference with other light of the same wavelength. At the same time, light also consists of particles, so-called photons. This duality is quite fundamental: the Hanbury Brown and Twiss experiment for example only works because of the particle-like properties of light.
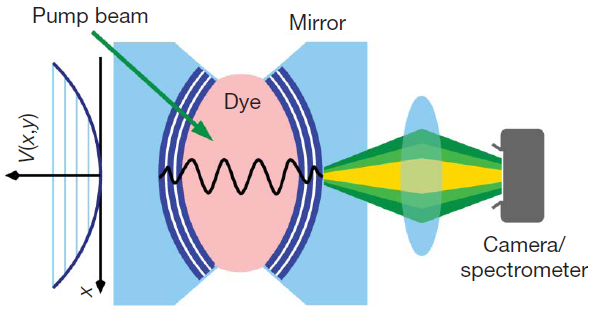
The experimental setup. A laser beam injects photons into a cavity filled with light, and a camera observes the photons coming out of the cavity. Reprinted by permission from Macmillan Publishers Ltd. Nature 468, 545-548 (2010).
This amazing and perhaps confusing duality, where light in one experiment appears to be a wave and in others it behaves like particles, is now laid bare in a paper published in Nature. There, Jan Klaers, Martin Weitz and colleagues from the University of Bonn in Germany take one of the classical properties of light waves and turn it upside down — by demonstrating a related effect that only works when considering the particle qualities of light!
The classical effect they use is that light waves can all oscillate synchronously. This is exactly what happens in a laser, and is typical behaviour for a class of particles to which photons belong to, the bosons. Bosons love to be all in the same state.
A similar synchronous behaviour can also occur for other bosons, including certain atoms, which then all assume the same quantum state. This state is called a Bose-Einstein condensate, after Satyendra Nath Bose (after whom bosons are named) and Albert Einstein, who described it first in 1924. It is a Bose-Einstein condensate of light that Weitz and colleagues have now demonstrated. […]
Continue reading...
If you look at the image of an atom in a text book, it looks rather quiet and peaceful. There is a nucleus in the center made from a number of protons and neutrons. Around the nucleus the electrons typically are shown to orbit the core like planets around the sun.

Cover of the 5 August 2010 issue of Nature (c) nature publishing group
The reality, however, is far more complicated. First of all, the electrons don’t look like small planets, but are smeared out in complex shapes known as orbitals. The energy states of the different orbitals correspond to the electron shells in atomic physics. And secondly the electron motion is extremely fast, on the timescale of a femtosecond (fs), which is 10-15 seconds. This is so short that even light can’t move very far in such a short time. Within a femtosecond it travels only about a third of a micrometer. So the question is, how is it possible to take a snapshot of such a fast motion? Well, one needs to use a camera that is even faster.
This ‘camera’ is a laser with attosecond resolution. An attosecond (as) is a thousand times faster than a femtosecond. In the present case, pulses of 150 as were used. These lasers are seriously fast. So fast that the light wave in the laser pulse completes only about one cycle. A bit like a tsunami wave that consists of only one big up and downwards motion of the water. Attosecond lasers were pioneered among others by Ferenc Krausz, with the clear aim of using them to venture into the intriguing realm of attosecond science. And it is his group that has now accomplished another major feat: the imaging of electron motions in the outer orbitals of krypton atoms. Their study appears in this week’s Nature, where it was also chosen for the cover.
[…]
Continue reading...




November 24, 2010
2 Comments