Your desk at work, is it chaotic as mine, or clean and ordered? If the latter, I salute you, because it takes work to keep a desk tidy. Otherwise, chaos will soon reign. And while I admit that I should keep my desk cleaner (and no, I won’t share photos here), I have an excellent excuse: it is a fundamental law of nature that disorder and chaos are always increasing.
A measure of this disorder is a quantity called entropy. To clean your desk it takes work, which creates heat, which is energy that is wasted on the environment. So even though the entropy of the desk is reduced, overall it increases. And this is the second law of thermodynamics: on average, the entropy in the universe is always increasing, whatever the process.
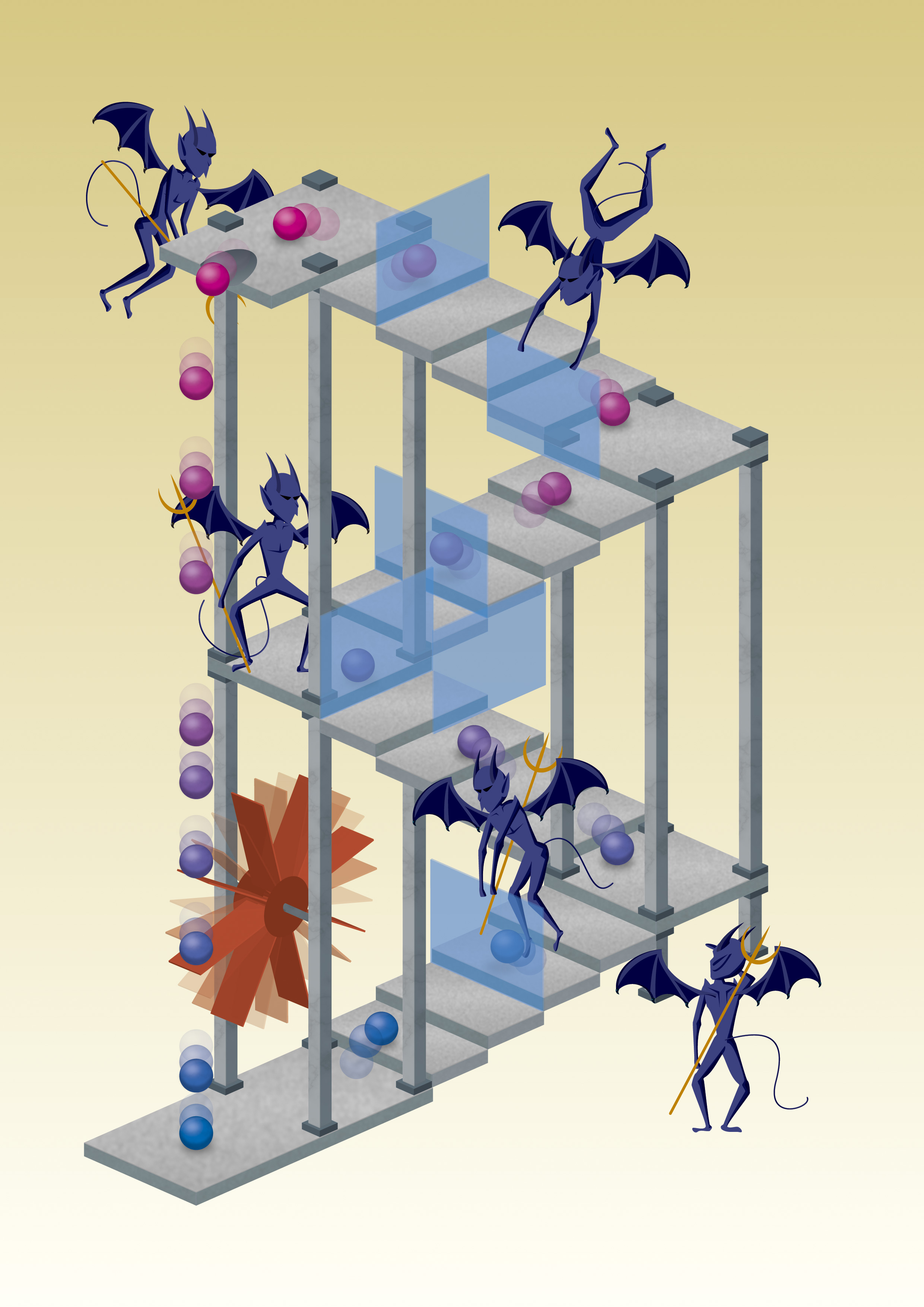
Maxwell's demon uses thermal motion of particles to let them move up a staircase and then blocks their way back. The effect is the same as with a box: particles on top of the stairs are warm, and cold at the bottom. Credit: Mabuchi Design Office / Yuki Akimoto
Well, asked James Clerk Maxwell back in 1867, what if you have a box filled with gas of a certain temperature. The box is separated into two compartments by a wall that has a small door. The door is controlled by a small ‘demon‘ that lets fast-moving gas molecules go into the right half, and leaves slow ones in the left. The left box would cool down, and the right one heats up. Overall, the box is more ordered than before. If the demon itself doesn’t use up any energy (which can be done), entropy would decrease, right? But according to the second law of thermodynamics energy is needed to create order, and the demon wouldn’t use any. Is this then a violation of the second law?
Well, actually not. The reason is that there is energy in information. To store a bit of information a system like a computer memory needs to be put into a defined state, either a ‘1’ or a ‘0’. This reduces entropy. And the same is true for the two compartments in a box. We use the information whether a molecule is fast or slow to separate them. The energy stored in that information is used to reduce the entropy of the system. So we are fine, the second law won’t be violated.
The energy that is contained in the information is tiny. For a single bit the lower limit is on the order of 10-21 joules. In comparison, one calorie, the old energy unit often used for food, corresponds to 4.184 joules.


November 14, 2010
4 Comments